The World Health Organization (WHO) recommends that typhoid conjugate vaccines (TCVs) be used in outbreak settings. TCVs have successfully been implemented during outbreaks in Pakistan and Zimbabwe; however, current data are limited in how and when to best introduce TCVs in response to outbreaks.
My team and I recently published an analysis that modeled different scenarios to try and answer this question. We developed a model of typhoid transmission fitted to data from a hospital in Blantyre, Malawi, from January 1996 to February 2015. The model accurately captured the multi-year outbreak of typhoid that occurred in Blantyre between 2011 and 2015.
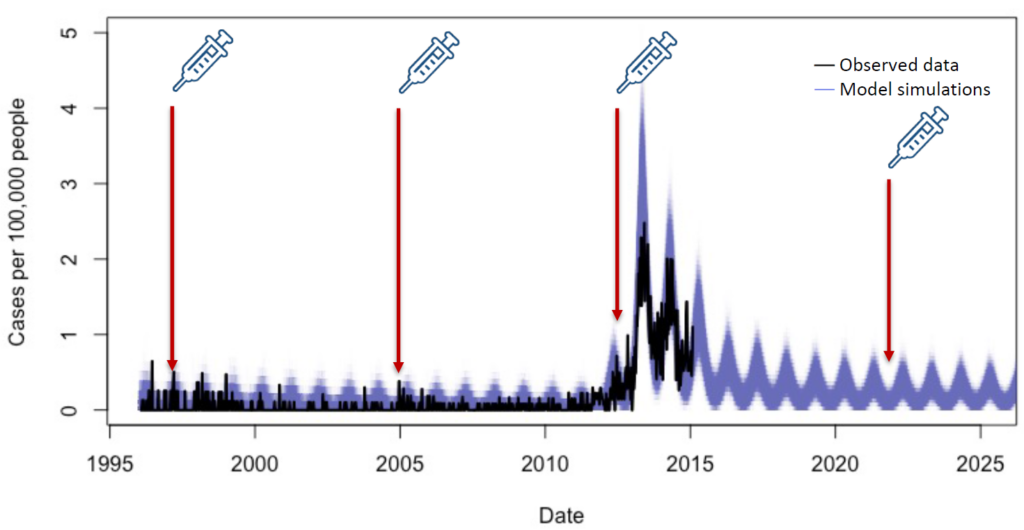
The model
We used our model to evaluate the cost-effectiveness of TCV vaccination strategies during a 10-year time period for three scenarios:
- When an outbreak is likely to occur in the next 10 years. (There are typhoid cases but an outbreak hasn’t been officially designated yet.) e.g. assuming TCVs were available and introduced between 2005-2010 in Malawi;
- When an outbreak is unlikely to occur within the next 10 years. e.g. if TCVs were available and introduced in 1996; and
- When an outbreak has already occurred and is unlikely to occur again. e.g. if TCVs were introduced today, with no further outbreaks occurring.
We then considered three potential TCV vaccination strategies compared to no vaccination:
- Preventative routine vaccination at 9 months of age;
- Preventative routine vaccination plus a catch-up campaign up to 15 years old;
- Reactive vaccination at 9 months of age with a catch-up campaign up to 15 years old.
Being proactive is usually the optimal vaccination strategy
The results from our analysis showed that for the most part, the preferred strategy from a cost-effectiveness standpoint is to introduce TCVs proactively before the outbreak occurs, as long as an outbreak occurs sometime in the next 10 years. In this situation, TCV introduction can considerably blunt the epidemic, although it does not prevent it completely. Reactive vaccination is only the preferred strategy if the delay to recognizing the outbreak and introducing TCVs is short and the willingness to pay for TCVs is low—less than US$300 per disability-adjusted life year (DALY) averted. DALYs are a measurement of overall disease burden expressed as the number of years lost due to ill-health, disability, or early death from a disease. This means it is usually more cost-effective to introduce TCVs before an outbreak occurs.
Assuming an outbreak occurs within 10 years of TCV introduction, our results estimated that the different vaccination strategies evaluated would prevent 15-60% of DALYs due to typhoid. It was always more cost-effective to introduce preventative routine TCV vaccination with a catch-up campaign to 15 years old. Routine vaccination with a catch-up campaign was the preferred strategy at willingness to pay values over $140 per DALY averted if an outbreak has already occurred, or above $890 if no outbreak occurs. Below these willingness-to-pay thresholds, TCV introduction was not cost-effective. Note that $140 is less than a quarter of the Malawi per capita gross domestic product (GDP; $635), whereas $890 is higher than the GDP. In other words, TCV introduction is likely to be cost-effective at the higher post-outbreak typhoid incidence seen today, but not at the earlier pre-outbreak typhoid incidence, if an outbreak does not occur.
Using data for decision-making
During disease outbreaks, reactive vaccination is a common preventative measure to help curb the spread and end the outbreak quickly. The results from our analysis show that reactive vaccination can be cost-effective, but only if delays in vaccine deployment are minimal. If delays in recognizing and responding to the outbreak persist for more than 6 months, introduction of preventive routine immunization with TCVs along with a catch-up campaign is the most cost-effective strategy.
Cost is often a major factor when policymakers decide whether to introduce a new vaccine in their country. These modeling results add to existing evidence on the cost-effectiveness of TCVs. They are particularly useful for countries that may have lower typhoid incidence but experience outbreaks frequently or are in regions with ongoing outbreaks. Furthermore, the rapid rise and spread of drug-resistant typhoid can seed more difficult to treat outbreaks. Multi-year typhoid outbreaks have occurred in many settings following the introduction and spread of drug-resistant strains. We hope that the findings from our analysis help inform country decision-making for TCV prevention and control strategies in outbreak settings, and in nearby countries at risk for future outbreaks.
Cover photo: Children wait to receive TCV as part of Pakistan’s outbreak response campaign in 2018. Photo: PATH/Asim Hafeez



