The currently available diagnostics in low- and middle-income countries present significant challenges in diagnosing typhoid fever. The gold standard for typhoid testing, blood culture, is expensive and requires specific laboratory equipment, skilled personnel, and resources which are often unavailable in typhoid-endemic settings. Even in places where blood cultures are available, they correctly identify only 40–60% of cases, leaving many people with typhoid undiagnosed. Most importantly, in many countries, blood cultures require 2-4 days to obtain results, which limits their clinical usefulness, making it difficult for healthcare providers to make timely treatment decisions and ensure proper patient follow-up.
The Widal test, a widely used rapid diagnostic for typhoid, is simple and inexpensive and provides results within minutes. However, its reliability is poor, leading to inaccurate diagnoses. The World Health Organization does not recommend the Widal test due to these limitations, which further restrict the diagnostic options available to clinicians in limited-resource settings.
Faster and more accurate point-of-care diagnostics are crucial for ensuring timely typhoid diagnosis and appropriate treatment, especially in limited healthcare settings. Delayed treatment increases the risk of severe complications, such as typhoid intestinal perforation (TIP), a life-threatening emergency requiring surgical intervention. Additionally, inaccurate diagnoses can lead to inappropriate antimicrobial treatments, further exacerbating the growing challenges of drug resistance.
Improving diagnostic accuracy will also provide a clearer understanding of the typhoid burden, enabling decision-makers to plan and implement more effective typhoid prevention and control strategies, including prioritizing the introduction of typhoid conjugate vaccine (TCV) in the routine immunization program.
Typhoid diagnosis in Bangladesh
Bangladesh is a typhoid-endemic country. About 290 typhoid cases per 100,000 people occurred in 2021, with 61% of cases affecting children under 15. However, the actual burden is likely underestimated due to difficulties in surveillance and diagnostics. The most commonly used diagnostic method in Bangladesh is blood culture.
In the absence of blood culture or when results are delayed, suspected typhoid cases are often treated based on clinical symptoms, which can easily overlap with other febrile illnesses. This increases the risk of misdiagnosis, inappropriate antibiotic usage, and developing complications like TIP.
A potential new rapid diagnostic
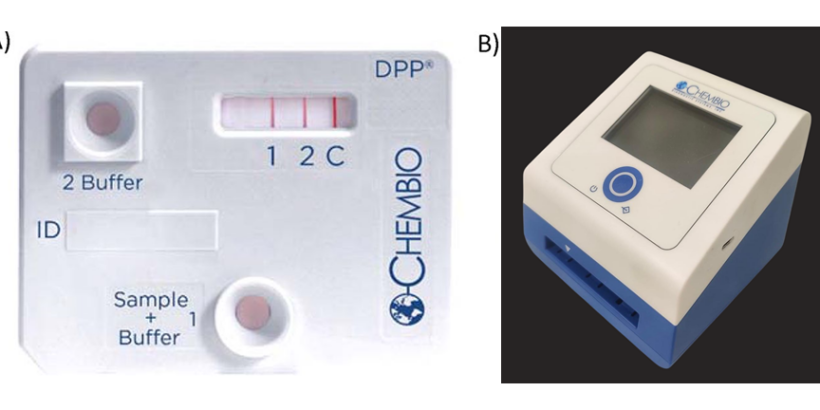
We conducted a prospective study in Dhaka, Bangladesh, to evaluate the accuracy of a new typhoid diagnostic called the dual-path platform for typhoid (DPPT) assay. This study collected venous blood, capillary blood samples via fingerstick, and nasal swabs from children under 18 with at least three days of fever.
The balanced accuracy of the DPPT assay (91%) outperformed other diagnostic methods, including blood culture (81%) and commercially available rapid tests such as Widal (70%). for diagnosing enteric fever. Latent class analysis showed that the DPPT assay had a sensitivity of 93% and specificity of 89%. With results available in 15 to 20 minutes, this assay provides clinicians with timely information to guide treatment decisions. The estimated cost is US$2 per test cassette with a one-time price of $250 for the microreader. The DPPT assay represents a significant advancement in addressing the challenges of typhoid diagnosis.
Although these results are promising, further studies are needed to evaluate how the DPPT assay performs in older age groups and diverse geographical locations with varying levels of enteric fever incidence. The continued development of the DPPT assay could improve clinical management, facilitate more appropriate antibiotic use, and help estimate the typhoid burden in communities where blood culture surveillance is not readily available.
Photo: DPP Typhoid Assay. The DPP Typhoid Assay consists of a test cassette, which consists of a sample path and reagent path that intersect in the analyte detection area labeled 1 (LPS), 2 (HlyE), and C (control) (A), and a DPP Micro Reader, a portable, battery-powered instrument that displays a numerical intensity value for the test lines. The DPP Micro Reader II is displayed (B). Credit: Kumar S, et al., 2020. Evaluation of a rapid point-of-care multiplex immunochromatographic assay for the diagnosis of enteric fever. mSphere 5:10.1128/msphere.00253-20 (A), and Child Health Research Foundation Laboratory, Dhaka, Bangladesh (B).